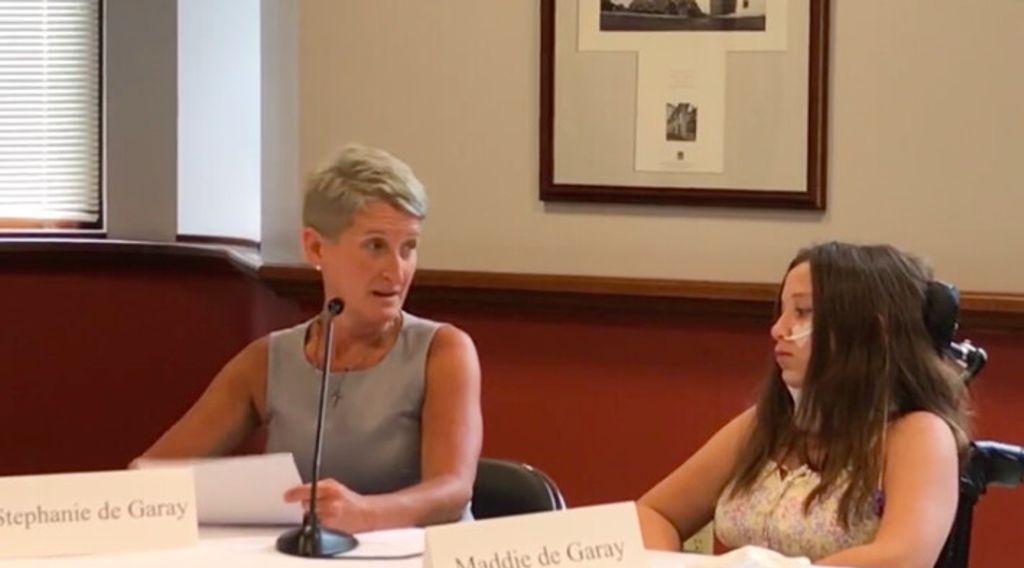
Maddie de Garay was a healthy, happy, straight-A student from Ohio who enjoyed hanging out with friends before she received her second dose of the Pfizer COVID-19 vaccine at the age of 12.
The now 13-year-old was one of the 2,260 voluntary participants in the Pfizer COVID-19 vaccine trial for adolescents aged 12 to 15 that began in July 2020. Maddie’s mother, Stephanie de Garay, said her daughter asked to participate in the trial as a way to help end the pandemic.
“My husband works in the medical field, and I have a degree in electrical engineering,” said de Garay, who spoke on behalf of her daughter at a press conference hosted by Sen. Ron Johnson (R-Wisc.) on June 28. “We are pro-vaccine and pro-science, which is why we agreed to let Maddie and her two older brothers volunteer for the trial.”
Johnson held a news conference to allow people from across the country who’ve experienced severe adverse reactions following a COVID-19 vaccine to share their stories after being “repeatedly ignored” by medical professionals and federal health authorities, advocating for Americans’ health care freedom.
According to de Garay’s testimony, which has been posted on an independent website by a group of “pro-vaccine, pro-science” Americans dedicated to raising awareness about rare cases believed to be COVID-19 vaccine injuries, Maddie received her first injection on Dec. 30, 2020, and experienced mild adverse reactions of fever, tiredness, and swelling at the injection site that resolved within a couple of days. However, after getting her second dose on Jan. 20, Maddie immediately felt pain at the injection site that didn’t occur with the first dose.
About 18 hours later, she developed “severe muscle/nerve pain, painful electrical shocks down her neck and spine which caused her to walk hunched over, severe chest pain that felt like her heart was being pulled out, numbness and swelling in her vaccine arm (left), her fingers and toes turned white and were ice cold to the touch, the pain in her toes was so bad she walked on her heels, severe abdominal pain (especially on [the] lower right side), and a fever of 101.4,” de Garay wrote.
Maddie was taken to the emergency room at the children’s hospital where the Pfizer vaccine trial was conducted.
“Her blood was taken for a renal profile and tested, she was checked for appendicitis, which she did not have, and given an IV with some medicine and sent home,” de Garay said. “In the discharge papers from the children’s hospital ER that she went to, the diagnosis stated adverse effect of vaccine initial encounter. This would be the only time that that was written in her medical charts.”
Maddie’s condition didn’t improve but worsened over the next 2 1/2 months as she developed more symptoms, including not being able to walk; inability to feel below her waist; memory loss; loss of bladder control; inability to swallow food or liquids, thus requiring a nasogastric feeding tube; and fainting or seizing 10 or more times per day.
“All these symptoms are still here today. Some days are worse than others,” de Garay tearfully said.
BY MEILING LEE