The U.S. Food and Drug Administration (FDA) on March 1 said that three rapid COVID-19 tests shouldn’t be used because of the potential for producing false results.
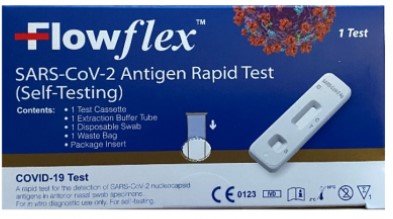
The FDA told people to avoid using the Celltrion DiaTrust COVID-19 Ag Rapid Test, the SD Biosensor Inc. STANDARD Q COVID-19 Ag Home Test, and the Flowflex SARS-CoV-2 Antigen Rapid Test (Self-Testing).
“The FDA is concerned about the risk of false results when using” those tests, according to the agency. These tests have “not been authorized, cleared or approved by the FDA for distribution or use in the United States,” the agency said.
All three tests work via nasal swab, the agency added. It recommended that health care providers have patients submit to new testing if they’ve used any of the three tests in the past two weeks.

The FDA said ACON Laboratories has recalled all of its Flowflex tests, SD Biosensor has recalled its tests, and Celltrion USA has recalled all of its DiaTrust tests.
“People should not use the Celltrion DiaTrust COVID-19 Ag Rapid Test that is in green and white packaging,” the FDA said, including a photo of the test.

SD Biosensor’s “unauthorized test may be packaged in a white and magenta box,” the FDA said.
ACON Laboratories’ tests are packaged in a dark blue box, according to the agency.
The FDA said it has “not received reports of injuries, adverse health consequences, or death associated with use of” any of the three tests.
In a statement, ACON Laboratories stated that the unauthorized tests are an “adulterated and misbranded counterfeit product.”
In February, the FDA issued warnings about the E25Bio COVID-19 Direct Antigen Rapid Test, the Empowered Diagnostics CovClear COVID-19 Rapid Antigen Test, and ImmunoPass COVID-19 Neutralizing Antibody Rapid Test for similar reasons. Recalls were also initiated for the tests.