
STORY AT-A-GLANCE
- One paper compared Merck’s data on molnupiravir against peer-reviewed data on ivermectin and found ivermectin has a low side effect profile, costs less than molnupiravir and is more effective against SARS-CoV-2
- Clinical Trials data show Merck gathered 1,850 participants but released data on only 762 in the non-hospitalized arm of the study. The study with hospitalized patients anticipated 1,300 participants, but enrolled 304 before terminating for “business reasons”
- Merck has applied for emergency use authorization for molnupiravir against COVID-19. Some are excited about an antiviral that may be effective against the virus, but the exclusion criteria for participants in the study may mean few will qualify to take the drug
- For many, prophylaxis and early treatment do not require prescription medication. Optimize your vitamin D level to help prevent the illness, and use nebulized hydrogen peroxide after exposure or in combination with nutraceuticals for early treatment
In the video above retired nurse lecturer John Campbell, Ph.D., reports on a comparative analysis of molnurpirivir and ivermectin published in the Austin Journal of Pharmacology and Therapeutics.1 The first is Merck’s new antiviral drug and the second is the much vilified and maligned2,3 antiparasitic drug used in humans since 19874 and approved for human use in the U.S. in 1996.5,6
Campbell compares the efficacy, safety and cost using available data for ivermectin published in peer reviewed studies and the first interim data for molnupiravir published by Merck. Molnupiravir, also known as EIDD-2801/MK-44827 has data published as early as October 2019 that showed it was a clinical candidate for monotherapy in influenza viruses.8
And yet, Merck’s investigation into the oral antiviral medication against SARS-CoV-2 was not logged with Clinical Trials until October 5, 2020.9 While Gilead raced to release remdesivir, posting their first clinical trial February 5, 2020,10 Merck appeared to be slow off the mark. Gilead suspended or terminated the early trials for remdesivir. The reasons given included:
- “The epidemic of COVID-19 has been controlled well at present, no eligible patients can be recruited.”11
- “The epidemic of COVID-19 has been controlled well in China, no eligible patients can be enrolled at present.”12
The advantage molnupiravir has over remdesivir is that it is administered orally and can be used for early treatment in an outpatient setting. However, as we review the comparison between the drugs, it’s important to remember that the early data on molnupiravir has been published in a press release.13
How Do Ivermectin and Molnupiravir Stack Up Against COVID-19?
In the video Campbell reviews a paper published in the Austin Journal of Pharmacology and Therapeutics14 that was a chemical comparison of the pharmacological effects of molnupiravir and ivermectin. Looking at the two ways science uses to develop new treatments when a new condition arises,15 Campbell explains the first is to create a new drug and the second is to repurpose medications used for other conditions.
For example, aspirin originally was used to treat fever. Once it became evident that it was also effective against pain, doctors began recommending it to relieve headaches and other minor aches and pains. Subsequently, it was found that aspirin was an effective antiplatelet, as well, and this function was added to the known uses for aspirin.
According to the paper,16 Ivermectin is the “most studied, ‘repurposed’ medication globally, in randomized clinical trials, retrospective studies and meta-analysis.” Ivermectin is an FDA-approved, broad spectrum antiparasitic17 with known anti-inflammatory properties.18
As Campbell reviews, an in vitro study19 demonstrated that a single treatment with ivermectin effectively reduced viral load 5,000 times in 48 hours in cell culture. By comparison, Merck claims molnupiravir is a broad-spectrum antiviral that is active against the Gamma, Delta and Mu SARS-CoV-2 variants.20
The data in the comparison paper show molnupiravir is more potent in-vitro than ivermectin,21 which means it needs less drug to work with a lower tissue concentration.22 The amount of time the maximum drug dose is found in the serum is one to 1.75 hours for molnupiravir and four to six hours for ivermectin.
Interestingly, the half-life for Merck’s drug is seven hours and the half-life for ivermectin is 81 to 91 hours. This is the amount of time it takes for your body to reduce the active ingredients in the drug by half. Campbell also reviews the following factors:
•Safety — No matter how well a drug works, if it’s not safe for use, it cannot be effective. Offering some examples of how ivermectin’s safety compares to other drugs, according to Campbell23 the global database of the World Health Organization, VigiBase, recorded 5,593 adverse events from ivermectin after 3.7 billion doses were administered to humans.
For comparison, VigiBase recorded 136,222 adverse events for amoxicillin and 165,479 for ibuprofen. At this time there is no VigiBase data available for molnupiravir, so no comparisons can be made for that drug yet. To take the example one step further, an outside look at acetaminophen adverse events shows that this drug (aka Tylenol) is many times more dangerous than ivermectin.
In the U.S. alone24 the National Institutes of Health’s STATPearls manual reports that there are 2,600 hospitalizations, 56,000 emergency room visits and 500 deaths each year for acetaminophen overdoses as of July 2021. And, the drug is the second leading cause of liver transplantation worldwide and the leading cause of transplantation in the U.S.
•Efficacy — According to interim data from Merck,25 molnupiravir reduced hospitalizations or deaths by 50% in 385 participants who had at least one risk factor associated with poor disease outcome. A meta-analysis of 15 trials26 that included 2,438 participants demonstrated that ivermectin could reduce the risk of death by 62%.
According to an ongoing collection from published data,27 across all studies ivermectin is 86% effective prophylactically, 66% effective in early treatment and 36% effective in late treatment. By comparison, a Cochrane review of the literature28 that Campbell references in the video found the data did not determine if ivermectin leads to more or less infections, worsened or improved infection, or increased or decreased unwanted events.
•Cost — According to a Forbes report,29 the raw material for the active pharmaceutical ingredients in molnupiravir costs about $2.50 per treatment. The cost of manufacturing the product would be $20, which is 35 times less than the price set by Merck of $700 per treatment. Additionally, Forbes reports that initially the drug will be purchased using federal funds.
According to the treatment protocol by the FLCCC,30 ivermectin is dosed at 0.4 to 0.6 mg/kg of body weight per dose once daily for five days. For an average person 160 pounds (72.5 kg), the dose is 29 mg to 43.5 mg per day for five days.
The average cost for 30 tablets of 3 mg of ivermectin in the U.S. can run as high as $108 or as little as $29.72 with a drug discount program — a fraction of molnupiravir’s prices.31
Peer Reviewed Study May Answer Molnupiravir Questions
As I mentioned, according to the data released by Merck, molnupiravir reduced the risk of hospitalization or death by 50% as compared to the placebo group.32 According to the numbers in their study, 28 people in the intervention group died or were hospitalized by Day 29 while 53 in the placebo treated group were hospitalized or died.
Merck did not identify the placebo in either their press release33 or in the Clinical Trials data.34 Dr. James Lyons-Weiler also evaluated the results of the trial and asked some very pertinent questions, such as:35
•Why were patients taking a placebo allowed to die?
“When there is a vast amount of published research on clear winners are the early treatment protocols as described by the medical authorities on the matter? Merck and NIH allowed 14.1% of people in the control arms to develop severe COVID-19 and die with no treatment. None. Just placebo.
How did the NIH and the FDA let this happen in the face of the evidence of efficacy of early treatment? How could they? Because that’s the standard of care for early COVID-19: go home, incubate, get sick, and die if you must. But don’t call us until you are seriously ill.”
•Why are the number of participants low? — When the study was first listed on Clinical Trials36 the team initially anticipated 1,450 patients in a parallel phase 2/3 randomized, placebo-controlled study. This changed May 25, 2021, to 1,850 participants anticipated.37
At the completion of the study when they were no longer recruiting participants, they reported data on 762 participants in the press release38 from 173 locations. What happened to the data from the rest of the participants?
•Why was the second study for hospitalized patients terminated? — A second study39 was ongoing during the same time period for hospitalized patients, having started October 5, 2020, and last updated September 9, 2021.
They anticipated enrolling 1,300 patients but terminated the study for “business reasons” after enrolling 304. What happened to cause the company to close this arm of the study after enrolling so few patients and what happened to the data?
Lyons-Weiler is a senior research scientist at the University of Pittsburgh.40 He also listed the numerous exclusion criteria for participants in the study and went on to write:41
“If, by any stretch of reason, FDA approval is made using the one interim analysis of (potentially) cherry-picked data in a cherry-picked study published as a press release without peer review, ignoring the data from the study not mentioned at all- their guidance should carry restrictions disallowing the use of the drug on or by patients in all of the excluded groups, including those who are hospitalized.
If by some miracle the rules on full reporting are enforced for the buried molnupiravir trial, the identified data from the trials need to be audited to make sure patients with an undesirable outcome under one trial were not excluded because they were enrolled in another trial focused on studying that same outcome. That would point to more scientific chicanery, and we’ve all had more than enough of that.”
CBS News42 reports that Merck has asked U.S. regulators for emergency use authorization for the drug against COVID-19. The decision could come in just a few weeks and “The FDA will scrutinize company data on the safety and effectiveness of the drug, molnupiravir, before rendering a decision.” It is hoped the FDA has access to all the data.
Do We Really Need a Vaccine and a Treatment?
Although Campbell adamantly defends the need for both a vaccine and treatment,43 he also points to diseases such as the bubonic plague for which we have adequate treatment but do not have a vaccine,44 even for areas of the world where it may have greater incidence.45
Campbell also believes that if there is a good quality antiviral medication, there would be less of an impact from COVID in countries where the vaccine rollout is patchy.
And yet, data show that the number of confirmed cases of COVID in countries where much of the population is unvaccinated is not higher than in countries where nearly 100% have been given the jab. For example, as of October 13, 2021, according to the CNN COVID-19 vaccination tracker46 and the Johns Hopkins Coronavirus Resource Center:47
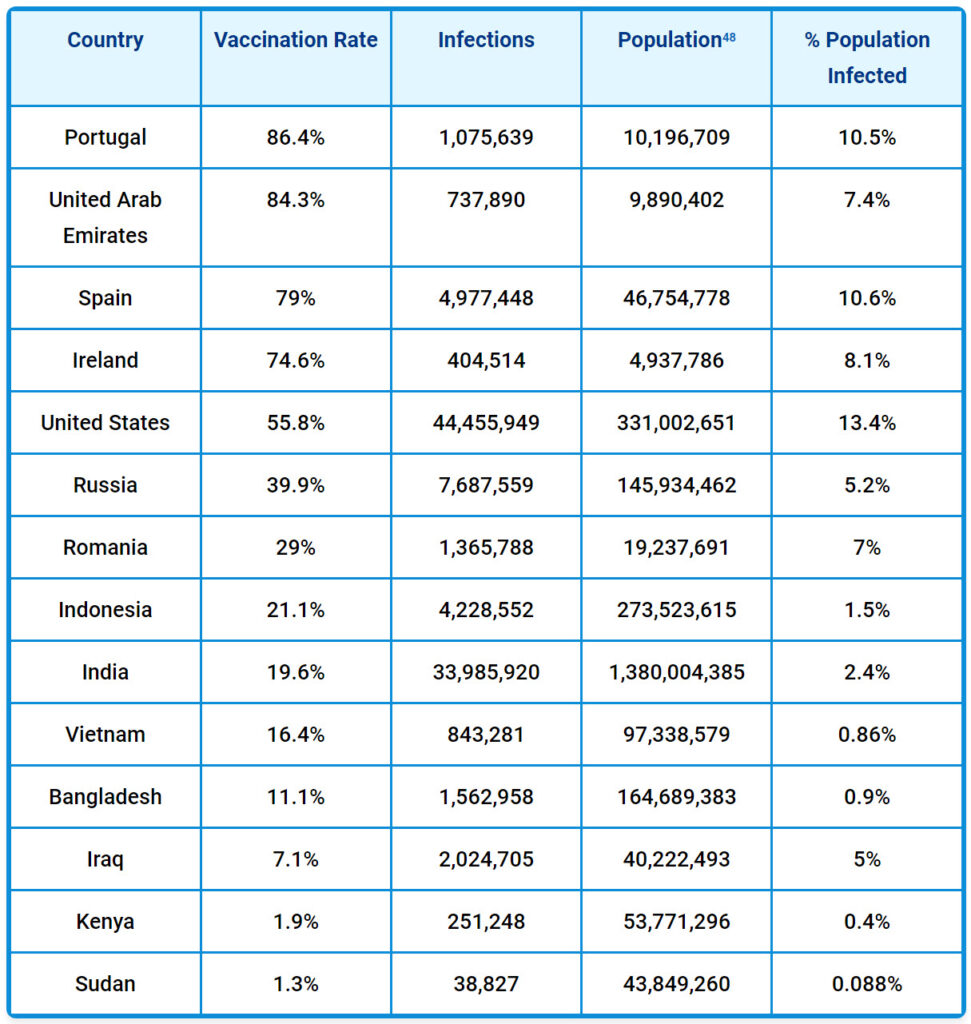
In the past, according to the CDC’s definition, a vaccination program used a product that “stimulates a person’s immune system to a specific disease, protecting the person from that disease.”49 But today, CDC’s new definition says vaccines are only meant to “stimulate the body’s immune response against diseases.”50 You’ll note that the new definition says a vaccine isn’t responsible for stimulating the immune system or protecting against any specific illness.
According to COVID-19 statistics from the CDC,51 people over 65 carry the greatest burden of mortality. In 2020 this population accounted for 80.7% of deaths and thus far in 2021 this age range accounts for 71.2% of deaths in the U.S. However, these percentages are highly skewed since, to date, large populations of people are not offered or treated with successful protocols.
This begs the question: How high has the CDC and FDA allowed the death rate to go by suppressing effective treatments that are readily available and economical?
Prophylaxis and Early Treatment May Not Require Medication
While ivermectin has demonstrated it is a useful strategy, it’s not my primary recommendation. You don’t necessarily need prescribed medication to help prevent, and in the early treatment of, COVID-19.
I believe your best option to fighting the onset of any disease is to optimize your vitamin D level, as your body requires this for a wide variety of functions, including a healthy immune response.52,53 Then, for early treatment, or after you’ve been exposed to someone with COVID, I recommend using nebulized hydrogen peroxide treatment.54
This treatment is inexpensive, highly effective, can easily be done at home and is completely harmless when you’re using the low (0.04% to 0.1%) peroxide concentration recommended. In the video below I demonstrate how to make a low concentration of hydrogen peroxide at home and how to use your nebulizer. You’ll find my interviews with Dr. Thomas Levy55 and Dr. David Brownstein56 about this treatment on Bitchute.
Sources and References
- 1, 14 Austin Journal of Pharmacology and Therapeutics, 2021; 9(5)
- 2 Business Insider March 1, 2021
- 3 Technician September 15, 2021
- 4 AAPS J. 2008 Mar; 10(1): 42–46
- 5 FDA Center for Drug Evaluation and Research September 23, 1996
- 6 American Chemical Society, Discovery of Ivermectin
- 7 Antimicrobial Agents and Chemotherapy, 2021;65(5)
- 8 Science of Translational Medicine, 2019;11(515)
- 9, 34 Clinical Trials, Identifier NCT04575597
- 10, 11 Clinical Trials, Identifier NCT04252664
- 12 Clinical Trials, Identifier NCT04257656
- 13, 20, 25, 32, 33, 38 Merck, October 1, 2021
- 15 YouTube, October 5, 2021, Minute 3:13
- 16 Austin Journal of Pharmacology and Therapeutics, 2021; 9(5) Introduction para 1
- 17 Journal of Medicinal Chemistry, 1980;23(10)
- 18 Archives of Bronconeumol, 2020;56(12)
- 19 Antiviral Research, 2020;178(104787)
- 21 Austin Journal of Pharmacology and Therapeutics, 2021; 9(5) page 1, rt col, line 7-9
- 22, 23, 43 YouTube, October 5, 2021
- 24 STATPearls Acetaminophen Toxicity July 18, 2021
- 26 American Journal of Therapeutics, 2021;28(4)
- 27 C19 Early
- 28 Cochrane Library, 2021; Ivermectin for Preventing and Treating COVID-19
- 29 Forbes, October 8, 2021
- 30 FLCCC, IMask+ DOWNLOAD
- 31 Good Rx, Ivermectin 3mg pills
- 35, 41 Popular Rationalism, The Extraordinary Hipocracy of Molnupiravir
- 36, 37 Clinical Trials, History of Changes for Study: NCT04575597
- 39 Clinical Trials, Identifier NCT04575584
- 40 Journal of Data Mining in Genomics and Proteomics, James Lyons Weiler
- 42 CBS News, October 11, 2021
- 44 Cleveland Clinic, Bubonic Plague
- 45 Centers for Disease Control and Prevention, Plague
- 46 CNN, October 13, 2021
- 47 Johns Hopkins Coronavirus Resource Center
- 48 Worldometer, World Population, Scroll near bottom and search tool on world population by country
- 49 CDC July 15, 2015
- 50 Centers for Disease Control and Prevention, Immunization the Basics, September 1, 2021
- 51 Centers for Disease Control and Prevention, Weekly Updates by Select Demographic and Geographic Characteristics, Table 1 yearly data
- 52 Nutrients, 2020, 12(5)
- 53 Nutrients, 2020;12(11)
- 54 Evidence-Based Complementary and Alternative Medicine July 3, 2021
- 55 Bitchute, April 2, 2021
- 56 Bitchute, February 17, 2021
Analysis By Dr. Joseph Mercola






