Last month, Revolver exclusively published a paper by Navy CDR J.H. Furman, warning that the mandatory COVID-19 vaccination of the entire Navy could constitute a national security threat.
Now, Furman has produced another paper, describing what he calls a “shell game” by which other U.S. government agencies, and several pharmaceutical companies are tricking the Navy into embracing mandatory vaccination, at great risk to the United States.
His paper is reproduced below.
CDR Jay Furman, USN
At the turning point of the Spanish American War, a single American officer volunteered to hand carry a critical message through impossible enemy lines to a fateful ally named General Garcia, forever changing the course of that war and our country. Today, one million COVID-19 non-vaccinated brave military messengers would deliver a, no less, existential dispatch: the U.S. Military is being misled by the U.S. Food and Drug Administration (FDA), the Center for Disease Control (CDC), and the Pfizer and BioNTech drug companies, resulting in the current mandatory COVID-19 vaccination policies. While some media outlets reflexively cheer on the deadly shell game, is our military leadership prematurely mandating a vaccine that their personnel cannot, or should not, legally receive? Could that decision prove to be more detrimental to military readiness than the disease itself, thereby posing a greater threat to U.S. national security?
On August 23, 2021, news broke that Pfizer had obtained an FDA license for its COVD-19 vaccine. The contrived FDA announcement:
Today, the U.S. Food and Drug Administration approved the first COVID-19 vaccine [BioNTech’s COVID-19 Vaccine, mRNA, not Pfizer]. The vaccine has been known as the Pfizer-BioNTech COVID-19 Vaccine [Pfizer’s non-FDA licensed vaccine], and will now be marketed as Comirnaty (koe-mir’-na-tee) [only available in markets outside U.S.], for the prevention of COVID-19 disease in individuals 16 years of age and older (emphasis added).
The agency charged with the public’s health in pharmaceutical matters issued two related official letters confounding the announcement, above. Reconciling those letters and the ensuing policy is tedious, leaving the truth one-layer too deep for most media outlets. Unfortunately, the resulting confusion appears more goal than incidental. Bottom-line, the FDA did not license a COVID-19 vaccine physically available to consumers in the U.S.
In their first letter, the FDA issued the only COVID-19 vaccination license for COVID-19 Vaccine, mRNA (Comirnaty) owned by German company BioNTech (not Pfizer). It is not produced for a U.S.-licensed label anywhere in the FDA’s jurisdiction. In their second letter, the FDA re-issued the Emergency Use Authorization (EUA) for Pfizer-BioNTech COVID-19 Vaccine (not a FDA license). The letter officially designates Comirnaty as the licensed name for COVID-19 Vaccine, mRNA. This EUA explicitly states that Pfizer-BioNTech COVID-19 Vaccine “[…] has not been approved or licensed by the FDA, but has been authorized by emergency use [EUA] by the FDA […]” (emphasis added). It goes on to assert that Pfizer-BioNTech COVID-19 Vaccine and Comirnaty (COVID-19 Vaccine, mRNA) formulations are the same and thereby can be clinically substitutable. The FDA notes the abundant supply of Pfizer-BioNTech COVID-19 Vaccine and the non-availability of Comirnaty (COVID-19 Vaccine, mRNA) in the U.S. market. They then explicitly re-affirm that Pfizer-BioNTech COVID-19 Vaccine is, in fact, experimental and only covered by the EUA, not FDA licensed.
FDA License Letter:


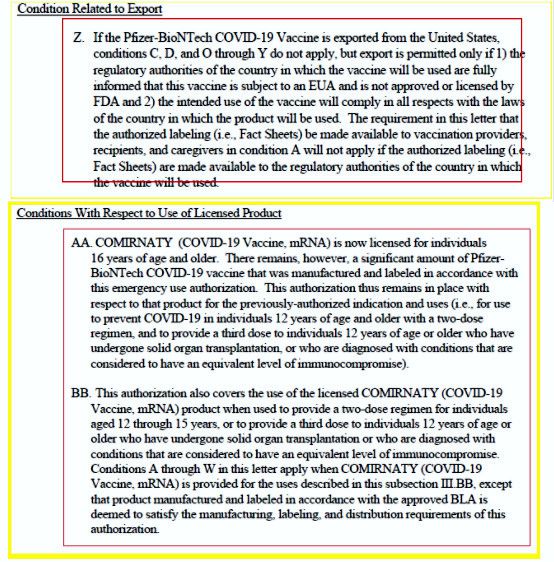
Confused yet? The CDC did not help matters, either. On Monday, August 31, 2021, their Advisory Committee on Immunization Practices (ACIP) released an inaccurate statement. The committee’s public announcement unanimously endorsed the FDA license for the “Pfizer-BioNTech licensed vaccine.” They misstated the vaccine name and license holder, never mentioning the actual owner of the FDA license, BioNTech, conflating legal ownership of Comirnaty (COVID-19 Vaccine, mRNA) as Pfizer’s. They neglected to mention it is not available in the U.S. and therefore not possible for consumers here to receive. Those inaccuracies further aided the public misperception that Pfizer-BioNTech COVID-19 Vaccine is FDA licensed. Concurrently, two high-level career FDA officials resigned at the time of the CDC ACIP board announcement, citing frustration with overreach into FDA affairs by other parts of the Executive Branch.
The Pfizer company issued a “forward-looking” press release, that was also easily misinterpreted. Perhaps they were hoping the mundane went unnoticed, as the pharmaceutical giant intermingled complex terms and concepts: Pfizer, BioNTech, Comirnaty, COVID-19, mRNA, EUA, authorized, approved, licensed, manufacturer, legal owner, national markets, and etc. The corporate relationship between Pfizer and BioNTech is no less convoluted. The joint venture and vaccine names, also similar, only add to the confusion. All of which potentially prevented a clear understanding of important legal and regulatory nuances, and allowed a fearful U.S. public to believe an available vaccine was now regulatorily licensed for their safety and legal recourse, when in reality, it was not. In brief:
- BioNTech is the marketing arm of the two company enterprise in the U.S., Europe, and UK.
- Pfizer produced Comirnaty (COVID-19 Vaccine, mRNA) for BioNTech, while both submitted supporting material for the license.
BioNTech, alone, received an FDA license for Comirnaty (COVID-19 Vaccine, mRNA). - Both Pfizer-BioNTech COVID-19 Vaccine and Comirnaty (COVID-19 Vaccine, mRNA) were covered, in-part, by the re-issued EUA.
- Both Pfizer-BioNTech COVID-19 Vaccine and COVID-19 Vaccine, mRNA may be available in other countries. Yet, Comirnaty (COVID-19 Vaccine, mRNA) is not available anywhere under FDA jurisdiction.
- If and when this occurs, Comirnaty (COVID-19 Vaccine, mRNA) will be “[…] manufactured, filled, labeled, and packaged at Pfizer.”
- Both Pfizer produced vaccines are, at times, marketed as Comirnaty outside the U.S. While only COVID-19 Vaccine, mRNA) is officially recognized as Comirnaty in this country.
Recent U.S. military vaccine mandates look to be a direct result of the manufactured confusion. August 24, 2021 DoD guidance stated that the Department “[…] will only use COVID-19 vaccines that receive full licensure from the Food and Drug Administration (FDA) […]” (emphasis added). A fully FDA licensed COVID-19 vaccine is not available to U.S. service members. They are simply not able to legally comply with the DoD mandate.
By administrative and regulatory law, it appears that all public and private institutions are not allowed to mandate EUA medical products. In 21 U.S. Code 360bb-3-Authorization for medical products for use in emergencies for unapproved products (b)(2)(e)(1)(A)(ii)(III), it says that recipients have “[…] the option to accept or refuse administration of the product.” In the FDA’s own policy guidelines it is written that recipients “[…] have the option to accept or refuse the EUA product […].” Under U.S. Code 335(i)(4) and related regulations, “the informed consent process typically requires human subjects to agree to the receipt… upon a disclosure that the product in question is not yet FDA approved and that the receipt of such product is voluntary.” Informed consent is required to administer EUA vaccines with few exceptions.
The newly mandated Pfizer-BioNTech COVID-19 Vaccine is legally defined as an EUA and therefore cannot be mandated in the military unless informed consent is waived by a presidential waiver and, according to U.S. Code Section 1107a of Title 10 and DoDI 6200.02, only after meeting specific criteria. Two of these criteria apparently preclude its issuance in this case: 1) “[…] specified military operation presents a substantial risk that military personnel may be subject to a chemical, biological, nuclear, or other exposure likely to produce death or serious or life-threatening injury or illness […]” and; 2) “[…] no available satisfactory alternative therapeutic or preventive treatment in relation to the intended use of the investigational new drug.” In the first, a waiver of informed consent is limited to the support of a specific military operation. For the second, monoclonal antibody therapy is an FDA-authorized alternative COVID-19 treatment.
It does not matter that the FDA stated in their re-issued EUA, their website, or Fact Sheet that the vaccines are similarly formulated and can be clinically interchangeable. The simple fact is that the administration of an EUA vaccine, by law, requires informed consent. It is therefore illegal to mandate any of the three U.S. available COVID-19 vaccines that are not officially licensed. As the Department did not receive an informed consent waiver from the President to mandate the Pfizer-BioNTech CV-19 Vaccine, and the FDA licensed Comirnaty (COVID-19 Vaccine, mRNA) is not yet available in the U.S., it remains uncertain how the military can continue to incorrectly mandate COVID-19 vaccination.
Consequently, the Services’ mandatory vaccination orders are impossible to lawfully execute according to Uniform Code of Military Justice (UCMJ) Article 90. U.S. military officers take an oath to the Constitution, and not to those appointed over them in the event orders are unlawful (enlisted service members swear allegiance to both). An officer may find themselves duty bound to refuse an unlawfully mandated vaccination in support and defense of the law of the land, on the behalf of their troops.
Meanwhile in other countries, Pfizer has not provided any COVID-19 vaccines without explicit (and extreme) indemnity contracts in place. They are requiring not only protection from all future product harm civil lawsuits, but also protection from Pfizer’s “[…] fraud, gross negligence, mismanagement, failure to follow good manufacturing processes… or malice […].” The company is requiring some countries to fund foreign bank accounts, take out insurance, and put up sovereign assets such as their Embassies or military bases, according to global health law lecturer Mark Eccleston-Turner of the University of England and statnews.com.
The aforementioned regulatory charade may be a U.S. version of Pfizer’s COVID-19 global indemnity project. Unfortunately, the company has a history of misbranding “with the intent to defraud or mislead.” In a landmark DOJ case they were found guilty of a felony, and fined $2.3 billion (the largest such fine ever) for fraudulent marketing. Interestingly, the FDA receives almost half of its current funding from industry in the form of “regulatory fees,” a clear conflict of interest which should be strongly questioned.
If the vaccine is actually safe and effective, then why all this confusion? Why did they only license “Comirnaty (COVID-19 Vaccine, mRNA),” exclusively available outside of this country? Why did they not license the “Pfizer-BioNTech COVID-19 Vaccine,” the only label available in the U.S. Why does Pfizer not just import the approved label? Why are they so keen on some form of almost total indemnity everywhere their vaccines are available? Why are we told they are interchangeable, but their license and EUA are legally not? If what is in the vial is the same, then why the legal labyrinth? And what exactly was the cross-agency overreach into FDA licensing processes and why is there no formal Congressional inquiry?
Sen. Ron Johnson (R-Wis.) is asking questions. He recently wrote a letter to the FDA requesting “why they did not grant full licensure for the Pfizer-BioNTech vaccine that is already in use and available in the U.S., and how the agency will ensure that those being vaccinated under mandates will receive the FDA-approved version […].” Those concerned about our nation’s defense should consider asking their Congressmen and Senators to do the same.
The complex web of words, business structures, international legal agreements, and unforthright regulation may not collectively protect U.S. citizens. Rather, they enable a slight-of-hand scheme that increases global market-share, reduces expenses, and lowers potential legal exposure. All the while, this product (with no long-term studies and declining efficacy) is pushed on the consumer—at all costs—regardless of the potential harm. If the confusion were removed as it should be, informed citizens, their elected officials, and public servants would not stand for this carnival-like side show.
Nine months into the vaccination campaign, available evidence to-date does not look good for existing U.S. vaccines. The original “wild” version of SAR-CoV-2 is virtually dead and the increasingly immune variants dominate. Delta is highly contagious, but much less dangerous to the general population. The largest real-world analysis study, examining 700,000 records in Israel’s official health database, found that the COVID-19 vaccinated are 13 times more likely to be infected and 27 times more likely to demonstrate serious symptoms than those with recovered natural immunity. The Combined Vaccine Adverse Event Reporting System (VAERS), which tracks post-vaccination events for possible patterns, has through August recorded more than 600,000 events, including 81,000 serious or life-threatening events and about 13,000 deaths.
In contrast, as of 12 August, the COVID-19 mortality rate in the military was .001%, or 29 deaths in the almost 2.2 million-strong and exceptionally healthy U.S. service member population. Recent studies, meanwhile, indicate that teen boys and young men (the military’s dominant demographic) are more likely to suffer heart problems from vaccination than they are to be hospitalized from COVID-19 itself. It is not difficult at all to imagine that in a force as young and healthy as the military, universal vaccination could cause more harm than the disease itself.
We all, citizen, elected official and military, want to protect the Force, but the extent of national harm that could result from doing so in this way, with wrong or incomplete information, is staggering to contemplate. Industry, regulators, and media must be immediately cross-examined. We literally only get one shot at this, as these vaccines are irreversible experimental gene therapies. I have previously suggested a Department-wide safety pause to conduct further study, so as to not prematurely commit the entire U.S. fighting force to one permanent experimental group. Given all of the cross-agency confusion, Congressional inquiry may be necessary. A bipartisan body could better investigate the misperceptions informing national security decisions.
Until more is reliably known, DoD can still maintain a control group with almost half of the 2.2 million uniformed population still deciding not to vaccinate. We could easily commence prevention and treatment therapies like I-MASK+ currently used in nations around the world with great efficacy.
It would be a national calamity to “rush to failure” en masse with the entire U.S. troop strength, using vaccines that may be more harmful than the disease itself to this specific population entrusted with our nation’s defense. The pervasive misperception that Pfizer-BioNTech COVID-19 Vaccine is FDA licensed, thereby justifying mandatory vaccination policy, is a logical syllogism based on a false premise and, therefore, invalid. The FDA, CDC, Pfizer, BioNTech and the media must reveal what is under all of the “shells” for the sake of this Republic.
Many U.S. military service members are carrying these messages to Garcia in the strongest terms possible, by tendering resignations. I am aware of many seasoned officers and enlisted who have done so, or done the effective equivalent (early retirement or non-reenlistment). This mandatory vaccination decision may, in the end, squander billions in training and readiness. The sudden loss of even a fraction of the one million non-vaccinated force could expose critical capabilities at scale. The talent capable of separating, at this time, have completed all service obligations, are fully trained and highly experienced, constituting the best of our warfighter expertise and lethality. Many others are declining to visit the military recruiter’s office for the first time. The already difficult task of recruiting for our all-volunteer force may become nearly impossible with a mandatory vaccination policy.
The most tragic are those already in the Service who have a remaining obligation (contracts or enlistments) and cannot choose to leave for risk of legal or financial ruin. Continue down this path, and our military will almost certainly suffer a heretofore-unseen morale deficit, further reducing overall fighting capability. If we run off or demoralize fully half of our armed force, then the defense of this nation will be significantly crippled.
And for what? To protect 0.001% of those in service? We would wreck our military, just after wrecking our economy, at the worst possible time, when U.S. soft power and perceived hard power are arguably at their lowest levels in half a century. This unnecessary vaccine mandate comes at a moment when strategic competitors are demonstrably more capable, more aggressive, and more able to project their will against U.S. interests than ever before.
If the mandatory COVID-19 vaccine policy is truly a military readiness initiative, then the reality is it will cause a far graver impact to our national defense posture than this disease. America’s foes do not care the reason why our Soldiers, Sailors, Airmen, Marines, Coast Guard or Guardians are not on duty to prevent attack. Mandatory vaccination’s enterprise-level damage to recruiting, retention, and trust and confidence within the ranks could make us all more vulnerable than COVID-19 ever could alone. It has been said that this great nation can only be conquered from within. If the military self-inflicts a strategic sized wound (by persecuting half the troops), then it is possible we could gift this nation’s enemies our own mortal blow.
To review, the FDA licensed vaccine is not available to consumers in the U.S. and even DoD cannot mandate any emergency vaccine without a specifically conditional, presidential waiver of a service member’s informed consent. To do otherwise is unlawful. The way I see it, service members have three choices: 1) choose to receive the EUA shot; 2) submit a religious and/or medical waiver, or; 3) refuse the EUA shot, as only a fully licensed vaccine labeled Comirnaty, not available at your U.S. clinic, can be mandated at present.
As we do best, service members are helping service members. More information can be found at COVID-19 educational information hub: thecontrolgroup.us
Read Original Article on Revolver.News