A judge forced the FDA to release Pfizer’s clinical data and it’s worse than you can possibly imagine!
The FDA was forced by a judge to release clinical data on the COVID vaccines back in January and so 55,000 pages of documents were just released. The FDA had originally wanted to hide the data for 75 years and release it in 2096 because, of course, the FDA is basically engaged in a criminal conspiracy. The COVID vaccines should never have been approved. This was obvious from the very beginning when animal trials were skipped in the Trump Administration’s ill-fated “Operation War Speed.” And now it’s undeniably true. We have the clinical data, and it’s horrific.
Hiding out in one appendix is the clinical data for Pfizer’s vaccine — which lists 1,291 adverse side effects in alphabetical order. Let’s give you just the bad things that can happen to people who took the Pfizer vaccine that start with the letter “a” to enjoy:
1p36 deletion syndrome; 2-Hydroxyglutaric aciduria; 5’nucleotidase increased; Acoustic neuritis; Acquired C1 inhibitor deficiency; Acquired epidermolysis bullosa; Acquired epileptic aphasia; Acute cutaneous lupus erythematosus; Acute disseminated encephalomyelitis; Acute encephalitis with refractory, repetitive partial seizures ;Acute febrile neutrophilic dermatosis; Acute flaccid myelitis; Acute haemorrhagic leukoencephalitis; Acute haemorrhagic oedema of infancy; Acute kidney injury; Acute macular outer retinopathy; Acute motor axonal neuropathy; Acute motor-sensory axonal neuropathy; Acute myocardial infarction; Acute respiratory distress syndrome; Acute respiratory failure; Addison’s disease; Administration site thrombosis; Administration site vasculitis; Adrenal thrombosis; Adverse event following immunisation; Ageusia; Agranulocytosis; Air embolism; Alanine aminotransferase abnormal; Alanine aminotransferase increased; Alcoholic seizure; Allergic bronchopulmonary mycosis; Allergic oedema; Alloimmune hepatitis; Alopecia areata; Alpers disease; Alveolar proteinosis; Ammonia abnormal; Ammonia increased; Amniotic cavity infection; Amygdalohippocampectomy; Amyloid arthropathy; Amyloidosis; Amyloidosis senile; Anaphylactic reaction; Anaphylactic shock; Anaphylactic transfusion reaction; Anaphylactoid reaction; Anaphylactoid shock; Anaphylactoid syndrome of pregnancy; Angioedema; Angiopathic neuropathy; Ankylosing spondylitis; Anosmia; Antiacetylcholine receptor antibody positive; Anti-actin antibody positive;Anti-aquaporin-4 antibody positive; Anti-basal ganglia antibody positive; Anti-cyclic citrullinated peptide antibody positive; Anti-epithelial antibody positive; Anti-erythrocyte antibody positive; Anti-exosome complex antibody positive; Anti-GAD antibody negative; Anti-GAD antibody positive; Anti-ganglioside antibody positive; Antigliadin antibody positive; Anti-glomerular basement membrane antibody positive; Anti-glomerular basement membrane disease; Anti-glycyl-tRNA synthetase antibody positive; Anti-HLA antibody test positive;Anti-IA2 antibody positive; Anti-insulin antibody increased; Anti-insulin antibody positive; Anti-insulin receptor antibody increased; Anti-insulin receptor antibody positive; Anti-interferon antibody negative; Anti-interferon antibody positive; Anti-islet cell antibody positive; Antimitochondrial antibody positive; Anti-muscle specific kinase antibody positive; Anti-myelin-associated glycoprotein antibodies positive; Anti-myelin-associated glycoprotein associated polyneuropathy; Antimyocardial antibody positive; Anti-neuronal antibody positive; Antineutrophil cytoplasmic antibody increased; Antineutrophil cytoplasmic antibody positive; Anti-neutrophil cytoplasmic antibody positive vasculitis; Anti-NMDA antibody positive; Antinuclear antibody increased; Antinuclear antibody positive; Antiphospholipid antibodies positive; Antiphospholipid syndrome; Anti-platelet antibody positive; Anti-prothrombin antibody positive; Antiribosomal P antibody positive; Anti-RNA polymerase III antibody positive; Anti-saccharomyces cerevisiae antibody test positive; Anti-sperm antibody positive; Anti-SRP antibody positive; Antisynthetase syndrome; Anti-thyroid antibody positive; Anti-transglutaminase antibody increased; Anti-VGCC antibody positive; Anti-VGKC antibody positive; Anti-vimentin antibody positive; Antiviral prophylaxis; Antiviral treatment; Anti-zinc transporter 8 antibody positive; Aortic embolus; Aortic thrombosis; Aortitis; Aplasia pure red cell; Aplastic anaemia; Application site thrombosis; Application site vasculitis; Arrhythmia; Arterial bypass occlusion; Arterial bypass thrombosis; Arterial thrombosis; Arteriovenous fistula thrombosis; Arteriovenous graft site stenosis; Arteriovenous graft thrombosis; Arteritis; Arteritis coronary; Arthralgia; Arthritis; Arthritis enteropathic; Ascites; Aseptic cavernous sinus thrombosis; Aspartate aminotransferase abnormal; Aspartate aminotransferas increased; Aspartate-glutamate-transporter deficiency; AST to platelet ratio index increased; AST/ALT ratio abnormal; Asthma; Asymptomatic COVID19;Ataxia;Atheroembolism;Atonic seizures; Atrial thrombosis; Atrophic thyroiditis; Atypical benign partial epilepsy; Atypical pneumonia; Aura; Autoantibody positive; Autoimmune anaemia; Autoimmune aplastic anaemia; Autoimmune arthritis; Autoimmune blistering disease; Autoimmune cholangitis; Autoimmune colitis; Autoimmune demyelinating disease; Autoimmune dermatitis; Autoimmune disorder; Autoimmune encephalopathy; Autoimmune endocrine disorder; Autoimmune enteropathy; Autoimmune eye disorder; Autoimmune haemolytic anaemia; Autoimmune heparin-induced thrombocytopenia; Autoimmune hepatitis; Autoimmune hyperlipidaemia; Autoimmune hypothyroidism; Autoimmune inner ear disease; Autoimmune lung disease; Autoimmune lymphoproliferative syndrome; Autoimmune myocarditis; Autoimmune myositis; Autoimmune nephritis; Autoimmune neuropathy; Autoimmune neutropenia; Autoimmune pancreatitis; Autoimmune pancytopenia; Autoimmune pericarditis; Autoimmune retinopathy; Autoimmune thyroid disorder; Autoimmune thyroiditis; Autoimmuneuveitis; Autoinflammation with infantile enterocolitis; Autoinflammatory disease; Automatism epileptic; Autonomic nervous system imbalance; Autonomic seizure; Axial spondyloarthritis; Axillary vein thrombosis; Axonal and demyelinating
polyneuropathy; Axonal neuropathy;
You get the idea. There are 9 pages of side effects in small print.
You already know that children, especially young boys, can get myocarditis from the vaccines but you should add to that list the serious possibility of them getting: a brain stem embolism, acute kidney injury, cardiac failure, frontal lobe epilepsy, Hashimoto’s encephalopathy, herpes, interstitial lung disease, or Type 1 diabetes mellitus — just to pick a few very serious side effects from a very sobering list.
And don’t tell me that your chances are slim of getting injured. The U.S. government’s own database, the Vaccine Adverse Events Reporting System (VAERS), has over 1 million reports of “adverse events” to the new vaccines — with 24,000 events listed as “death.” Pfizer was aware of more than 158,000 “adverse events” when they asked for approval from the FDA. People had serious issues after taking the Pfizer vaccine and Pfizer knew it before it sought approval for its vaccine. Look at this chart compiled by Pfizer itself.
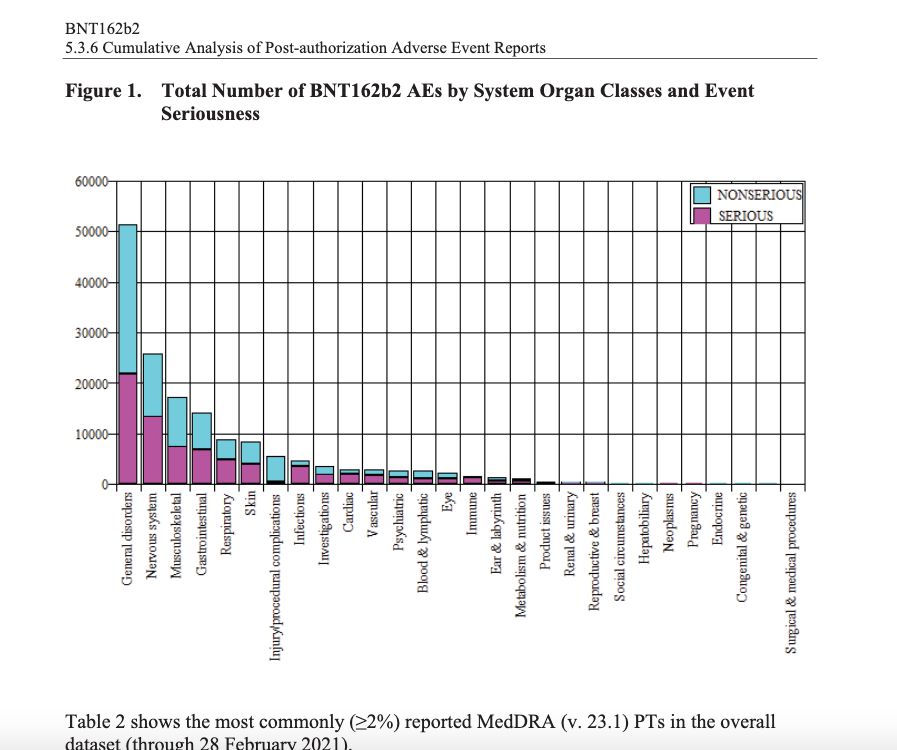
Why would the FDA approve a new vaccine when 15,000 people had serious disorders of the nervous system after taking it?
There’s simply no good reason.
Tell your friends and tell your family: the vaccination of children must stop immediately. The U.S. government has bought 50 million doses of this poison for children under the age of 5 pending FDA approval and it must never be allowed to use them.
Call your elected representatives, call your senators, call everyone you know to put a stop to this today.
Do not allow anyone to jab a child with this stuff.
Read Original Article on Emeralddb3.substack.com